Extraction of Lithium Using Electrode Materials of Lithium Ion Battery-II |ChemFam #54|
Greetings to everyone! In my previous blog, I have dived in to the Li recovery systems based on electrochemical methods. We have discussed the water-splitting battery system, asymmetric battery system as of now. Still, we are yet to discuss two other methods of the same. These are, as I have already mentioned in my previous blogs; hybrid supercapacitor system and symmetric rocking chair battery-liked system. Without further ado, let's continue from where we left.


Hybrid Supercapacitor System
The hybrid supercapacitor system represents a novel combination of the traditional supercapacitor and the Li-ion battery. It aims to harness the fast charge/discharge characteristics of supercapacitors while maintaining the high energy density of Li-ion batteries. This system employs a unique architecture, often integrating both electrode types with distinct charge storage mechanisms. By doing so, it seeks to offer improved power delivery, enhanced cycling stability and efficient energy utilization, thereby making it an attractive candidate for applications requiring rapid bursts of power and prolonged energy storage.
Recently hybrid supercapacitor systems have attracted a lot of attention. They not only borrow the advantages of Li-ion batteries, but also have good value double-layer capacitors. In general, a negative electrode can adsorb and desorb the ions in the electrolyte and act as the non-faradic capacitive cathode. Mostly, activated carbons suitable for aqueous solutions containing various anions are widely used in hybrid supercapacitor systems due to their environmental friendliness, economy and stability. For example, Kim et al showed a hybrid supercapacitor in which λ-MnO2 and activated carbon were matched.
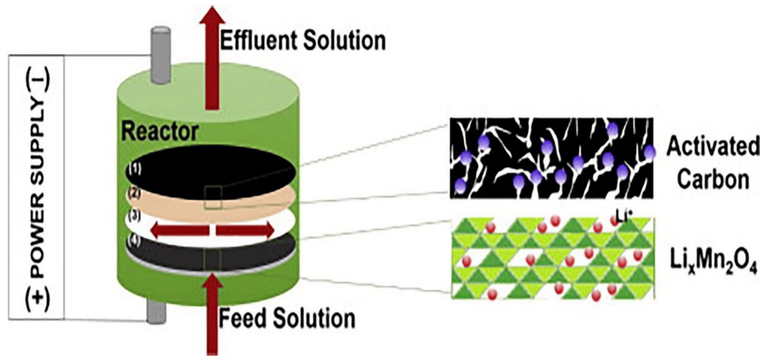
(1) an activated carbon-composite electrode, (2) an anion exchange membrane, (3) nylon as a spacer and (4) a λ-MnO2 composite electrode, are contained in the reactor.
Considering the adsorption of activated carbon to cations, an exchange mechanism must be introduced into the system. At the same time, it is logical that the exchange of Li ions remains stable after 50 operating cycles and exhibited long term stability. To avoid the use of corrosive acidic solutions in the static lithium treatment, Ryu et al. presented an improved membrane capacitive deionization process with an electric field assisted desorption system containing WE and LiMn2O4 as activated carbon. Compared with the traditional lithium desorption process, the electric field assisted desorption was expected to be a suitable and practical process for lithium recovery because of its low cost and low pollution properties. However, these systems face the problem of low resolution that should be mentioned.

Symmetric Rocking-Chair-Battery System
The symmetric rocking chair battery-liked system focuses on revolutionizing the conventional Li-ion battery design. Traditionally, Li-ion batteries employ asymmetric electrode materials (e.g., graphite anode and transition metal oxide cathode) that store Li-ions in a unidirectional manner during charging and discharging processes. The symmetric rocking chair battery-liked system, in contrast, employs identical materials as both positive and negative electrodes, leading to bidirectional Li-ion movement. This novel design not only simplifies the battery's structure but also facilitates faster ion transport, minimizing electrode degradation and offering a pathway towards achieving higher energy densities and faster charging rates.
Rechargeable lithium-ion batteries used in aqueous electrolytes are considered to be safe and cost-effective electrochemical lithium extraction system. In general, lithium can be transferred from brine to a recovery solution through the interaction and release at the electrodes with an electrical source (i.e,. condition of an applied voltage). Currently, the representative symmetrical rocking chair battery is LiFePO4/FePO4, in which the electrodes maintains the Li-saturation and Li-deficiency state. For example, Zhao et al. successfully extracted lithium from brines with high Mg/Li ratios which was achieved by using the LiFePO4/FePO4 system.

Generally, lithium intercalation and release takes place at the electrodes according to the metal oxidation and reduction processes. In addition, like LiFePO4/FePO4, LiMn2O4/Li1−xMn2O4 can be used as a rocking chair electrode system for lithium extraction. For example, Guo et al. fabricated a LiMn2O4/Li1−xMn2O4 system in which the adsorption and desorption of Li can be done simultaneously. More importantly, the effect of heteroatom ions on the Li extraction process is being also discussed. The results show that chloride ions have a positive effect on the lithium extraction process, while magnesium ions have a negative effect. Meanwhile, the difference in ion concentration near the cathode boundary layer causes an increase in the resistive potential, which reduces the Li extraction efficiency. In many cases, whisking the solution can reduce this negative effect. In addition, Zhao et al. reported a rocking chair battery-like system composed of LiMn2O4 and Li1−xMn2O4.
Co existing ions such as Mg2+, Na+, Ca2+, K+, etc. present in the electrolyte have a negative effect on the extraction of Li, among which magnesium ions have the greatest effect. Meanwhile, the voltage of 1.2V can produce maximum Li extraction capability; however, it also comes with the disadvantage of high power consumption. Depending on the change in the ion migration rate, the temperature also has a positive effect on the increase in the lithium extraction rate. Considering the effect of lithium extraction rate and specific energy consumption, 25°C was chosen as the appropriate temperature.

Although the above symmetric rocking-chair-battery-like system provides great flexibility in Li extraction from seawater, high ion-resistance caused by the anion exchange membrane leads to the high energy-consumption of the system, which needs to be further optimized.
Electrode Materials
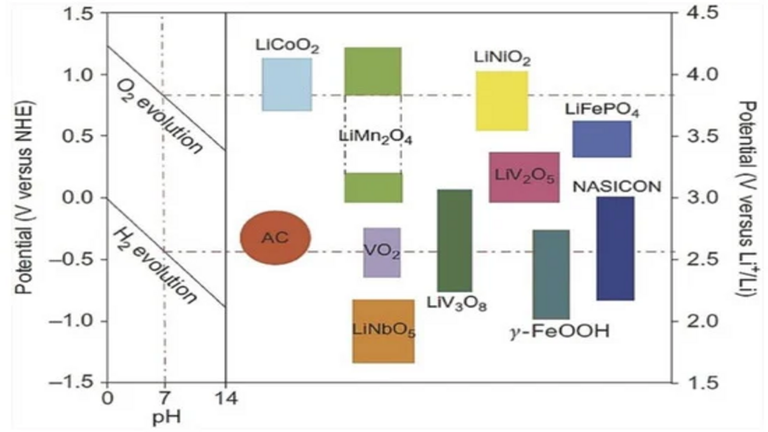
Electrochemical Li extraction is carried out using the principle of Li+ transfer between electrodes and electrolyte during charging and discharging of lithium-ion batteries (LIBs). Theoretically, any cathode or anode material available for Li-ion batteries can be used to extract Li from brine/seawater. Considering the stability, ease of preparation, low cost and environmental friendliness, various electronic materials can be chosen as electrode materials for Li recovery. This is mainly to introduce the new progress in electrochemical lithium extraction technology in recent years, and to present and review the introduction of LiFePO4 (LFP), LiMn2O4 (LMO) and other electrode materials.
LiFePO4 Based Materials
LiFePO4 (LFP) has an orthorhombic lattice structure with a space group of Pnma. Its lattice parameters satisfy a = 10.33 Å, b = 6.01 Å and c = 4.69 Å. Its structure can be simplified to consist of FeO6 octahedra and LiO6 octahedra, which are connected together by PO4 tetrahedra. Therefore, when the FePO4 is formed by delithiation, the skeleton structure is not changed. Moreover, it has been shown that LFP has a high stability, due to the strong interaction between the O atoms and Fe and P atoms, which can remain stable at high temperatures up to 400°C. In turn, LFP exhibits high cyclic-stability.
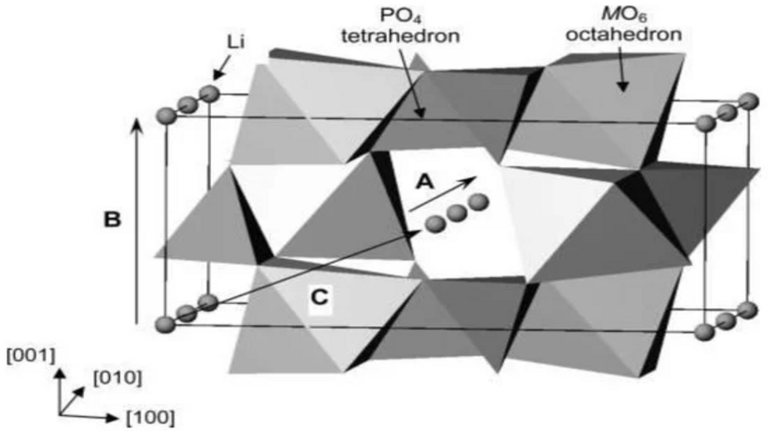
Recently, LFP as a transition-state salt of Li phosphate is proved to have the capability of inserting/detaching a Li+ between each iron atom, reversibly. This has become the most attractive electrode material for Li+. The reaction equations for LFP in the extraction process of Li+ is,
LiFePO4 ↔FePO4 + 𝐿𝑖+ + 𝑒−
Based on the probability of crystal phase transition, Pasta et al. first used LFP as a catalyst to selectively remove Li+ from ocean water. The battery can convert sodium-rich brine (Li+: Na+ = 1:100) into lithium-rich (Li+: Na+ = 5:1 ) solution through charging and discharging. As a comparison, the authors calculated that the energy consumption of the electrochemical lithium extraction process according to the method proposed by Kanoh et al. was only 33 W h mol-1; this shows that the energy consumption is less than previously reported data.
However, Ag was used as an anionic material in Pasta's experiment, which resulted in an expensive cost and the presence of Cl- in seawater accelerated the precipitation of the cathode electrode. Therefore, to reduce the cost and increase the stability of the reaction, Zhao et al. extracted Li+ by using FePO4 instead of Ag as the anionic material. Thanks to the special coating of FePO4, Li+ can be easily absorbed into the FePO4 electrode by the Li-containing solution. In addition, due to the reverse redox reaction of Li+ separation from the LFP material and Li+ intercalation to the FePO4 material, the cell voltage is obtained and the theoretical cell voltage of the LFP/FePO4 cell is calculated as zero. The results show that the intercalation capacity of lithium in pure lithium solution can reach 41.26 mg·(1 g LFP)-1, which is 93.78% of the theoretical value.
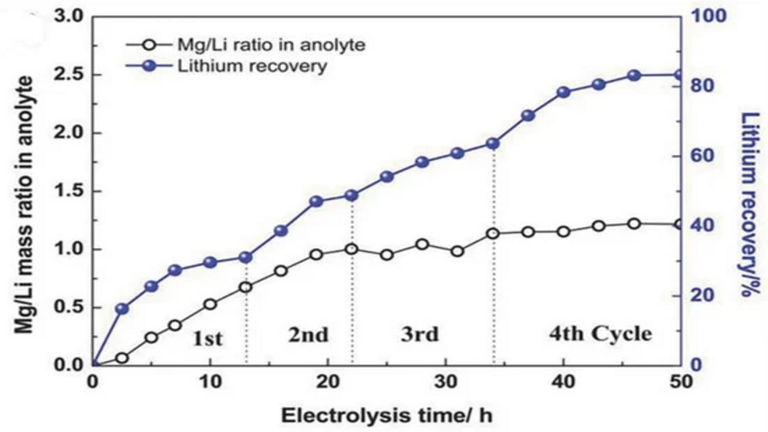
Professor Zhao's research group also used LFP/FePO4 as an electrode material to extract lithium to solve the global problem of lithium removal from high magnesium-lithium brine. The figure below shows the efficiency of Li extraction at different Mg2+:Li+ ratios (= 10, 20, 30, 60) using the electrochemical method. The results show that even in solutions with a high Mg2+ content (Mg2+: Li+ = 10), the withdrawal rate can be up to 83% as long as the low voltage (less than 0.75 V) is maintained during the pull-back operation.
.

Conclusive thoughts
Although LFP/FePO4 could be used as an electrode material for efficient Li acquisition, current studies have focused on high-Li-concentration brines and Li and Na concentrations ranging from 0.001 to 1. However, real seawater with a very low Li concentration has rarely been tested. Therefore, on the basis of using FePO4 material, Liu et al. plated a layer of hydrophilic TiO2 coating on the electrode surface to reduce the intercalation overpotential, further improving the Li-extraction efficiency in seawater with an extremely low Li/Na ratio. Even when the initial Li+/Na+ ratio reached 1.6 × 10−3, the recovery ratio of lithium to sodium reached more than 50:1 in 10 cycles. Meantime, the hydrophilic TiO2 coating increased the contact area between the electrode and electrolyte. In 10 Li-acquisition cycles, the material could still achieve a 1:1 Li-to-sodium ratio with a molar-ratio selectivity of up to 1.8 × 104.
As the demand for efficient and sustainable energy storage solutions continues to surge, the integration of advanced electrochemical methods like the hybrid supercapacitor system and the symmetric rocking chair battery-liked system could hold the key to meeting our evolving energy needs. By understanding the underlying principles and technological advancements in these systems, we can pave the way for a greener and more electrified future.
Until we meet again :).
Extraction of Lithium Using Electrode Materials of Lithium Ion Battery |ChemFam #53|
Helium: The First Noble Gas |ChemFam #52|
Hydrogen: The Simplest Atom |ChemFam #51|
Elements, Atoms and Atomic Theory |ChemFam #50|
Have You Thanked A Clod Today? |ChemFam #49|
Nuclear Energy: Will It Rise Again? |ChemFam #48|
SCRAP Giveaway | Terracore | Draw #10 |
Soaps: An Essential and Effective Cleansing Agent |ChemFam #47|
SCRAP Giveaway | Terracore | Draw #5 |
Chemicals in Food : Debunking Myths and Ensuring Safe Consumptions |ChemFam #46|
Unveiling The Secrets of Antiseptics and Disinfectants |ChemFam #45|
What are Antimicrobials and Antimicrobial Drugs? |ChemFam #44|
Therapeutic Action of Different Classes of Drugs |ChemFam #43|
Introduction to Drugs and Drug-Target Interaction |ChemFam #42|
Scientists Analyze a Single Atom With X-Rays For The First Time |ChemFam #41|
Can We Slow Down Aging? |ChemFam #40|
Studying The Cluster Compounds: The LNCC |ChemFam #39|
PS The thumbnail image is being created by me using canva.com by using template image from mdpi


Nice @splash-of-angs63
Yet another exciting episode of Lithium ion batteries. Keep it up 👍🏻
Thank you for the nice words @vinamra
They keep me motivated:)
This post has been manually curated by @bhattg from Indiaunited community. Join us on our Discord Server.
Do you know that you can earn a passive income by delegating to @indiaunited. We share more than 100 % of the curation rewards with the delegators in the form of IUC tokens. HP delegators and IUC token holders also get upto 20% additional vote weight.
Here are some handy links for delegations: 100HP, 250HP, 500HP, 1000HP.
100% of the rewards from this comment goes to the curator for their manual curation efforts. Please encourage the curator @bhattg by upvoting this comment and support the community by voting the posts made by @indiaunited.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.