Elements, Atoms and Atomic Theory |ChemFam #50|
Greetings to everyone! We know that chemistry is the science of matter. The basic building blocks of matter are atoms of various elements that are composed of subatomic particles, these are; positively charged protons (+), negatively charged electrons (-), and electrically neutral neutrons (n). It is the properties of these atoms that determine the chemical behavior of a substance. More precisely, the arrangement and energy levels of the electrons in an atom determine how the electrons interact with each other, which determines all chemical behavior. Today, we shall be looking at these elements from the perspective of basic building blocks of Green chemicals and also we shall discuss about the atomic theory as well.

One of the most fundamental aspects of chemistry is the periodic change in the behavior of elements as their atomic number increases. This has enabled placement of elements in an orderly arrangement with increasing atomic number known as the periodic table. The periodic behavior of elements’ chemical properties is due to the fact that, as atomic number increases, electrons are added incrementally to atoms and occupy so-called shells, each filled with a specific number of electrons. As each shell is filled, a new shell is started, thus beginning a new period (row) of the periodic table. This sounds complicated, and indeed may be so, occupying the full-time computational activities of banks of computers to explain the behavior of electrons in matter.
However, this behavior can be viewed in simplified models and is most easily understood for the first 20 elements using dots to represent electrons, enabling construction of an abbreviated 20-element periodic table. Despite its simplicity, this table helps you understand and explain many of the chemical phenomena. We shall be highlighting some environmental aspects of the first 20 elements and their relationship to sustainability. These elements include nitrogen, oxygen, carbon (which is contained in carbon dioxide), hydrogen, and oxygen (which is contained in water vapor), which make up most of the air in the "green" atmosphere. Hydrogen and oxygen in water are probably the most environmentally friendly compounds. Sodium and chlorine in table salt; silicon, calcium and oxygen which make up most minerals, including the soil in which plants grow that serve as food for most organisms and hydrogen, oxygen, carbon, nitrogen, phosphorus and sulfur that are predominant elements in all living material.
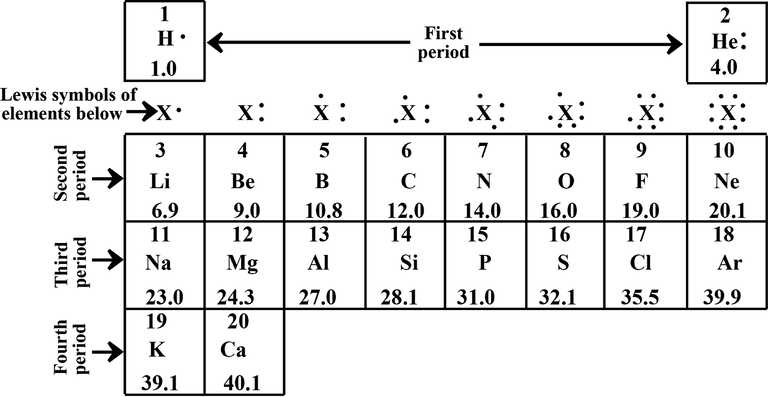
Long before subatomic particles were known, there was Dalton's atomic theory
The atomic theory explained the relationship between atoms for behavior. Chemists now have advanced equipment, and the properties of atoms (and most of the subatomic particles that compose them) are well known. But long before people imagined this complex and sophisticated instruments, nearly two centuries ago, in 1808, an English teacher named John Dalton came up with the atomic theory that bears his name. To a large extent, this theory is the basic principle of modern chemistry. The main points of Dalton's atomic theory are:
• Matter consists of tiny particles called atoms in all elements.(Dalton believed that atoms were indivisible and unchanging substances. We now know that they exchange and share electrons, which are the basis for the formation of chemical bonds.)
• Atoms of different substances have different chemical properties. (These differences can be as small as the difference between neon gas and argon, or as large as the difference between strong sodium metal and strongly non-metallic chlorine.)
• Atoms cannot be created, destroyed or changed to atoms of other elements.(Now, a provision has been added that these things do not happen in ordinary chemical processes, because atoms can become atoms of other elements through nuclear reactions, as in nuclear reactors.)
• Chemical compounds are formed from combinations of atoms of different elements in definite constant ratios, usually these fixed ratios can be expressed as numbers or simple fractions.
• Chemical reactions involve the splitting and combining of atoms. (This phenomenon seems to have been predicted long before the nature of the chemical bonds was known.)
The Three Fundamental Laws
Dalton's Atomic Theory explains the following three fundamental laws. Evidence of these laws were found before the publication of Dalton's atomic theory, which is largely based upon these laws.
1. Law of Conservation of Mass:
It is believed that mass does not change in ordinary chemical reactions. (This law was originally proposed in 1798 by the "Father of Chemistry" Antoine Lavoisier : In ordinary chemical reactions atoms cannot be lost, added or replaced and so this follows the conservation of mass.)
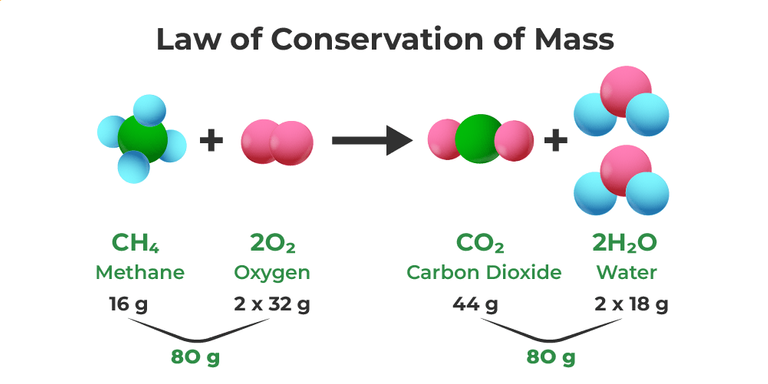
2. Law of Constant Composition:
A given chemical compound always has the same elements in the same proportions by mass.

3. Law of Multiple Proportions:
When two elements are mixed to form two or more compounds, the masses of one element that combines with the fixed mass of other element are in ratios of simple whole numbers. Examples of this principle are simple carbon and hydrogen containing hydrocarbon compounds such as CH4, C2H2, C2H4 and C2H6. In these compounds, the relative masses of C and H are in the ratio of small whole numbers.

Conclusive thoughts
The atomic theory laid the foundation of modern chemistry with the principles of the indivisibility of atoms, the conservation of mass and the formation of compounds from the reactions of various elements. It has paved the way for many ongoing research, emerging technological developments and innovations that has changed our world.
The study of elements and atoms will undoubtedly be the focus of scientific research as we move into the future. The knowledge we gain from the atomic realm will continue to improve humanity's progress, from solving the mysteries of the universe to the creation of new materials and medicines.
We shall meet again :)
Have You Thanked A Clod Today? |ChemFam #49|
Nuclear Energy: Will It Rise Again? |ChemFam #48|
SCRAP Giveaway | Terracore | Draw #8 |
Soaps: An Essential and Effective Cleansing Agent |ChemFam #47|
SCRAP Giveaway | Terracore | Draw #5 |
Chemicals in Food : Debunking Myths and Ensuring Safe Consumptions |ChemFam #46|
Unveiling The Secrets of Antiseptics and Disinfectants |ChemFam #45|
What are Antimicrobials and Antimicrobial Drugs? |ChemFam #44|
Therapeutic Action of Different Classes of Drugs |ChemFam #43|
Introduction to Drugs and Drug-Target Interaction |ChemFam #42|
Scientists Analyze a Single Atom With X-Rays For The First Time |ChemFam #41|
Can We Slow Down Aging? |ChemFam #40|
Studying The Cluster Compounds: The LNCC |ChemFam #39|
Biochemistry of Calcium: Role of Calcium in Muscle Contraction |ChemFam #38|
Biosynthesis of Fatty Acids: De Novo Synthesis of Fatty Acids |ChemFam #37|
Hapticity and The Eighteen Electron Rule |ChemFam #36|
An Introduction To Organometallic Chemistry |ChemFam #35|
PS The thumbnail image is being created by me using canva.com by using template image from Sciencenews


Congratulations @splash-of-angs63! You have completed the following achievement on the Hive blockchain And have been rewarded with New badge(s)
Your next target is to reach 25000 upvotes.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPCheck out our last posts:
wonderful explanation of the atomic theory. keep on.
Thank you bro :)
This post has been manually curated by @bhattg from Indiaunited community. Join us on our Discord Server.
Do you know that you can earn a passive income by delegating to @indiaunited. We share more than 100 % of the curation rewards with the delegators in the form of IUC tokens. HP delegators and IUC token holders also get upto 20% additional vote weight.
Here are some handy links for delegations: 100HP, 250HP, 500HP, 1000HP.
100% of the rewards from this comment goes to the curator for their manual curation efforts. Please encourage the curator @bhattg by upvoting this comment and support the community by voting the posts made by @indiaunited.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.
Congratulations your publication has been chosen among the best of the day.
KEEP CREATING GOOD CONTENT.
Thank you so much for the kind gesture :)