How light alters the rate of chemical reactions - with examples


Introduction
During a chemical reaction, elements or compounds combine to form new products. Whether such reactions happen in the laboratory or industrially, the speed of reaction is very important. There are chemical reactions that happen very fast and there are ones that are not so fast. Scientists understand the rates of chemical reactions and know how to control them in order to achieve the desired result. For example, if a chemical reaction needs to happen at a slower rate, then certain measures would need to be taken assuming the speed of reaction is naturally too fast.
So the rate of a chemical reaction is simply the speed at which the reactants combine to form the products. It is often measured in terms of how long it takes for the reactants or products to change quantitatively. For example, converting some moles of a certain element like oxygen to form a compound happens at a specific rate. Depending on what the desired results should be, this speed of reaction could be increased or decreased.

Controlling the rate of chemical reactions
Often, chemical reactions are managed in order to have the results expected. Most chemical reactions happen at rates that are not economically viable. For examples, some reactions are too fast for the desired products to be collected. In this case, the reaction is slowed down using any of the methods that would be described below. There are still other chemical reactions that happen so slowly that it would take a long time to have the products. Again here, the reaction could be sped up.
There are a number of ways or conditions that could slow down or increase the reactions rate of a chemical process. Take a look at just three of them:
Light and chemical reactions
The presence or absence of light during a chemical reaction has a great effect on its speed. Most, light speeds up the rate of most reactions at makes the reactants to be more vigorous as they combine with each other. Consider the following examples:
- Decomposition of Hydrogen Peroxide: Hydrogen Peroxide which is chemically represented as H202 can disintegrate to form oxygen gas and water molecules. The chemical reaction occurs more vigorously in the presence of light. In other words, light could be used to speed up the reaction.
The presence of light in the chemical decomposition of H202 causes the bonds between the oxygen molecules to break up quickly. Thus, the free oxygen radicals continue to react with more H202 causing the compound to decompose. The product thus becomes water molecules and oxygen. When this reaction occurs in the presence of light, there is often no need to add any chemical catalysts. Light alone would be enough to cause a direct and quick breakdown of the oxygen bonds to form the products.
The equation below represents the chemical reaction:

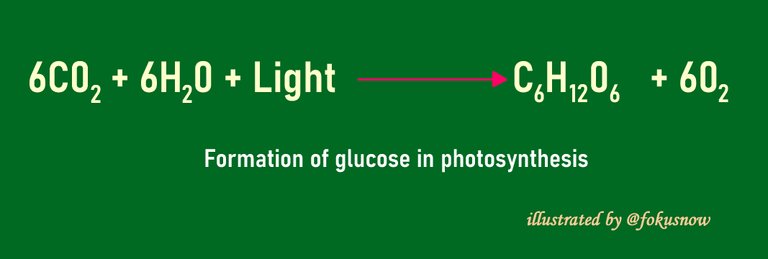
- Formation of glucose in Photosynthesis: Another chemical process in which lights affects the rate of reaction is photosynthesis. In this reaction, light does not just speed up the speed of reaction, it is an important condition that must be there in the first place, for the reaction to take place. At the end of the chemical combination, glucose is produced and used as plant food.
The energy needed to kick off this process is provided often by sunshine. The plant cells have special pigments that absorb the light from the sun and energizes the transfer of electrons inside the plant cells. The chemical reactions powered by this release of electrons ends up in the use of carbon dioxide in air to produce glucose. The reaction also releases some amounts of oxygen to the environment.
Photosynthesis happens more vigorously when the sunshine is stronger. The more the intensity of light, the more excited electrons released within the cell are, towards the formation of glucose in the cell. This is another chemical reaction in which light affects the reactants and products formed.
You can see below a chemical equation to illustrate the formation of glucose during photosynthesis.
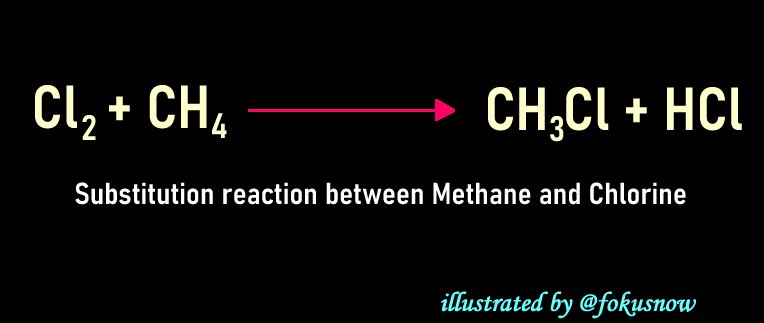
- Reaction between Chlorine and Methane: This is another reaction in which light plays a key role. Just like the above example of photosynthesis, light kicks of this reaction. There is a strong bond between the two atoms of the chlorine molecule. For this bond to be broken, the reaction must happen in the presence of light. Once the bond is broken by light, the liberated chlorine ions then reacts with methane molecules and displaces an atom of hydrogen. At the end, Hydrochloric acid is also formed in the process.
The chemical equation for the above reaction can be seen below:

Finally
The above chemical reactions are examples of the effect light can have to alter chemical reactions. In the presence of light, most reactions happen faster or more vigorously. And as shown in the photosynthesis example, light is needed for some reactions to happen at all.
Read more on this:
Note: Thumbnail from Pixabay. Other Images are mine!
Posted Using InLeo Alpha
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.